Please choose a vault from below or make a deposit...
n. Polythene Bag - Polyethylene or polyethene is a thermoplastic commodity heavily used in consumer products. Its name originates from the monomer ethene used to create the polymer. A Polythene bag is created using a machine called an extruder to blow air in to molten polymer to create a big bubble, the bubble is then collapsed flat to create layflat polythene film. This is then cut and sealed using bagmaker machines to create a Polybag.
Polythene bags are the main stay of the packaging industry and come in a vast array of shapes, sizes and strengths which include carrier bags, mailing bags, grip-seal bags, slider-grip bags, bubble-bags, garment bags and the good old bin bag. For each of these there are now whole equivalent ranges of biodegradeable bags as well. Please see the buyers guide below which helps address some common queries.
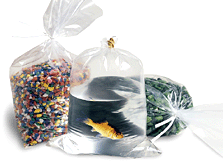
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bags
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bags
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bags
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bags
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bags
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bags
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bags
 Shopping > Packaging > polythene bags
Shopping > Packaging > polythene bagsThe most popular polythene bags vary in width from 2" (50mm) to about 48" (1220mm). These bags are normally stocked in multiple thicknesses - 120g (light duty use), 250g (medium duty use) and 500g (heavy duty use).
If you have a slightly unusual configuration in mind or need a custom size you can simple request a mnufacturer to make them for you.
In the standard format for describing a bag:
* the width of the bag is always taken to be the side that opens, so this is normally, but not necessarily, the shorter side.
* the width of the bag is given before the length.
* the thickness of the bag is given last and is usually expressed in 'gauge' (100 gauge = 1/1000 of an inch).
* hence the size of a bag is given as: Width (inches) x Length (inches) x Thickness (Gauge)
For example: if a bag is said to be 4" x 6" x 150 gauge then the bag is 4" wide by 6" long with 150 gauge thickness and the bag will open on the 4" width.
This is a crystal clear type of bag often used for greetings-card bags or for other display reasons.
In the polymer industry Polyethylene is sometimes shortened to PE, similar to how other polymers like polypropylene and polystyrene are shortened to PP and PS, respectively. In the United Kingdom the polymer is called polythene. (e.g. in the Beatles song Polythene Pam). The ethene molecule (known almost universally by its non-IUPAC name ethylene), C2H4 is CH2=CH2, Two CH2 connected by a double bond.
Polyethylene is created through polymerization of ethene. It can be produced through radical polymerization, anionic polymerization, and cationic polymerization. This is because ethene does not have any substituent groups which influence the stability of the propagation head of the polymer. Each of these methods results in a different type of polyethylene.
Polyethylene is classified into several different vaults based mostly on its density and branching. The mechanical properties of PE depend significantly on variables such as the extent and type of branching, the crystal structure, and the molecular weight.
* UHMWPE (ultra high molecular weight PE)
* HDPE (high density PE)
* HDXLPE (high density cross-linked PE)
* PEX (cross-linked PE)
* MDPE (medium density PE)
* LDPE (low density PE)
* LLDPE (linear low density PE)
* VLDPE (very low density PE)
Polyethylene was first synthesized by the German chemist Hans von Pechmann, who prepared it by accident in 1898 while heating diazomethane. When his colleagues Eugen Bamberger and Friedrich Tschirner characterized the white, waxy substance he had created, they recognized that it contained long -CH2- chains and termed it polymethylene.
The first industrially practical polyethylene synthesis was discovered (again by accident) by Eric Fawcett and Reginald Gibson at ICI Chemicals in 1933. Upon applying extremely high pressure (several hundred atmospheres) to a mixture of ethylene and benzaldehyde, they again produced a white waxy material. Since the reaction had been initiated by trace oxygen contamination in their apparatus, the experiment was at first difficult to reproduce. It was not until 1935 that another ICI chemist, Michael Perrin, developed this accident into a reproducible high-pressure synthesis for polyethylene that became the basis for industrial LDPE production beginning in 1939.
Subsequent landmarks in polyethylene synthesis have centered around the development of several types of catalyst that promote ethylene polymerization at more mild temperatures and pressures. The first of these was a chromium trioxide based catalyst discovered in 1951 by Robert Banks and John Hogan at Phillips Petroleum. In 1953, the German chemist Karl Ziegler developed a catalytic system based on titanium halides and organoaluminum compounds that worked at even milder conditions than the Phillips catalyst. The Phillips catalyst is less expensive and easier to work with, however, and both methods are used in industrial practice.
By the end of the 1950s both the Phillips and Ziegler type catalysts were being used for HDPE production. Phillips' initially had difficulties producing a HDPE product of uniform quality, and filled warehouses with off-specification plastic. However, financial ruin was unexpectedly averted in 1957, when the hula hoop, a toy consisting of a circular polyethylene tube, became a fad among teenagers throughout the United States.